Introduction to Eukaryotic Animal Cells
Eukaryotic animal cell coloring – The eukaryotic animal cell, a marvel of biological engineering, represents a complex and highly organized system responsible for the diverse functions of animal life. Its intricate architecture, a testament to millions of years of evolution, is characterized by a membrane-bound nucleus and an array of specialized organelles, each contributing to the cell’s overall survival and function. Understanding the structure and function of these components is crucial to comprehending the fundamental principles of animal biology.The eukaryotic animal cell is distinguished by its compartmentalization; its internal space is divided into distinct functional regions by a system of internal membranes.
This organization allows for the efficient and simultaneous execution of numerous biochemical processes, preventing conflicts and enhancing overall cellular efficiency. This sophisticated structure is in stark contrast to the simpler prokaryotic cells, lacking membrane-bound organelles.
The Nucleus: The Cell’s Control Center
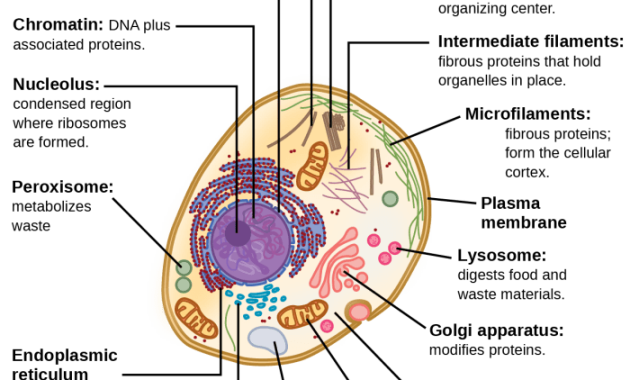
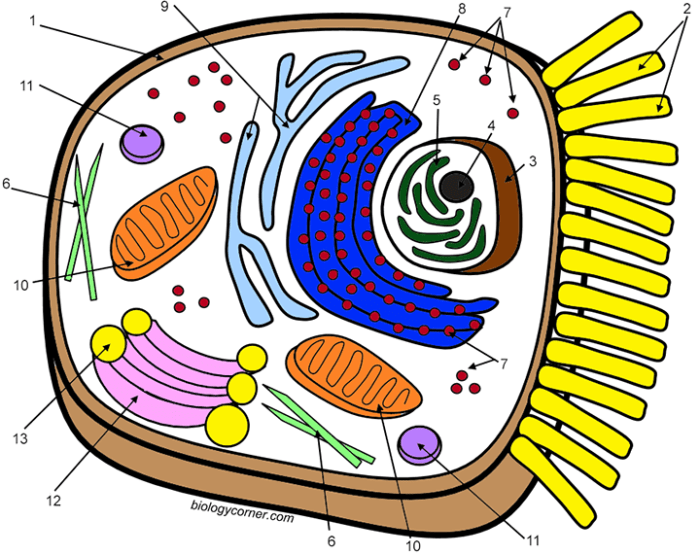
The nucleus, the cell’s most prominent organelle, houses the genetic material, DNA, organized into chromosomes. It acts as the cell’s control center, dictating the synthesis of proteins and regulating all cellular activities. The nuclear envelope, a double membrane perforated by nuclear pores, controls the passage of molecules between the nucleus and the cytoplasm. Within the nucleus, the nucleolus is a prominent structure responsible for ribosome biogenesis, the creation of the protein synthesis machinery.
The highly organized structure of chromatin, the complex of DNA and proteins, ensures efficient storage and accessibility of genetic information.
Mitochondria: The Powerhouses of the Cell
Mitochondria are often referred to as the “powerhouses” of the cell because they are the primary sites of cellular respiration, the process that generates ATP (adenosine triphosphate), the cell’s main energy currency. These double-membraned organelles possess their own DNA and ribosomes, remnants of their endosymbiotic origins. The inner membrane, folded into cristae, significantly increases the surface area available for ATP production through oxidative phosphorylation.
Mitochondrial dysfunction is implicated in a range of diseases, highlighting their critical role in cellular health.
Ribosomes: Protein Synthesis Factories
Ribosomes are complex molecular machines responsible for protein synthesis. These organelles, composed of ribosomal RNA (rRNA) and proteins, can be found free in the cytoplasm or bound to the endoplasmic reticulum. Free ribosomes synthesize proteins destined for use within the cytoplasm, while bound ribosomes produce proteins destined for secretion or insertion into membranes. The precise coordination of ribosome activity is essential for the synthesis of the vast array of proteins required for cellular function.
Endoplasmic Reticulum: A Network of Membranes
The endoplasmic reticulum (ER) is an extensive network of interconnected membranous sacs and tubules that extends throughout the cytoplasm. The rough ER, studded with ribosomes, is involved in protein synthesis and modification. The smooth ER, lacking ribosomes, plays a crucial role in lipid synthesis, detoxification, and calcium storage. The ER’s extensive network facilitates the efficient transport of proteins and lipids throughout the cell.
Golgi Apparatus: The Cell’s Processing and Packaging Center
The Golgi apparatus, also known as the Golgi complex, is a stack of flattened, membranous sacs called cisternae. It receives proteins and lipids from the ER, further modifies them, and sorts them for transport to their final destinations within or outside the cell. The Golgi apparatus adds carbohydrate chains to proteins, creating glycoproteins, and packages molecules into vesicles for transport to other organelles or secretion.
Its precise organization and function are essential for the proper delivery of cellular components.
Lysosomes: The Cell’s Recycling Centers
Lysosomes are membrane-bound organelles containing hydrolytic enzymes that break down cellular waste products, foreign materials, and damaged organelles. These enzymes function optimally at acidic pH, maintained by proton pumps within the lysosomal membrane. Lysosomal dysfunction can lead to the accumulation of cellular debris, contributing to various diseases. The lysosome’s crucial role in cellular recycling is essential for maintaining cellular homeostasis.
The Cell Membrane: Maintaining Cell Integrity
The cell membrane, a selectively permeable phospholipid bilayer, is the boundary that separates the cell’s internal environment from its surroundings. This dynamic structure, composed of phospholipids, cholesterol, and proteins, regulates the passage of molecules into and out of the cell. The phospholipid bilayer, with its hydrophilic heads and hydrophobic tails, forms a barrier that prevents the free passage of most molecules.
Membrane proteins, however, facilitate the transport of specific molecules, including ions and larger molecules, across the membrane. This selective permeability maintains the cell’s internal environment and is essential for its survival. The fluid mosaic model describes the membrane’s dynamic nature, with its components constantly moving and interacting. The cell membrane also plays a vital role in cell signaling and communication, receiving and transmitting signals from the external environment.
Maintaining the integrity of the cell membrane is crucial for cell survival and function.
Cell Components and Their Coloring
The visualization of eukaryotic animal cell components relies heavily on staining techniques. These techniques exploit the chemical properties of both the cellular structures and the stains themselves to selectively highlight specific organelles and cellular features under a microscope, enabling detailed observation and analysis of cellular morphology and function. The choice of stain is crucial, as different stains bind to different cellular components with varying affinities, resulting in distinct colors and levels of contrast.
The chemical basis of staining hinges on the interaction between the stain and the cellular components. Many stains are charged molecules; for instance, some are cationic (positively charged) dyes that bind to negatively charged cellular structures like nucleic acids (DNA and RNA) within the nucleus and other anionic components of the cytoplasm. Conversely, anionic dyes bind to positively charged cellular components.
Other staining methods rely on the lipid solubility of the stain, allowing it to penetrate and stain the cell membrane. The intensity of staining can also reflect the concentration of the target component within the cell, providing further insights into cellular processes.
Staining Methods for Different Cell Components
The visualization of the nucleus, cytoplasm, and cell membrane requires distinct staining approaches. Nuclear staining typically utilizes dyes with a high affinity for nucleic acids, enabling clear visualization of the nuclear envelope and chromatin structure. Cytoplasmic staining often involves dyes that interact with various cytoplasmic components, providing an overall picture of the cytoplasmic matrix and potentially highlighting specific organelles based on their chemical properties.
Cell membrane staining often employs lipophilic dyes that integrate into the lipid bilayer, thereby outlining the cell’s perimeter. The choice of stain and staining protocol influences not only the color but also the level of detail observable in each cellular component.
Comparison of Staining Techniques
The following table compares three common staining techniques used in animal cell microscopy:
| Staining Technique | Stain Used | Target Cell Component | Resulting Color |
|---|---|---|---|
| Hematoxylin and Eosin (H&E) staining | Hematoxylin (basic dye), Eosin (acidic dye) | Nucleus (Hematoxylin), Cytoplasm & Extracellular Matrix (Eosin) | Nuclei: Purple/Blue; Cytoplasm & ECM: Pink/Red |
| Wright-Giemsa staining | Methylene blue, Eosin, Azure B | Nucleus, Cytoplasm, Granules (depending on cell type) | Nuclei: Dark purple/blue; Cytoplasm: Varies (pink to light blue); Granules: Variable colors |
| Sudan Black B staining | Sudan Black B | Lipids (cell membrane, lipid droplets) | Black/Dark blue |
Illustrative Examples of Colored Cell Structures
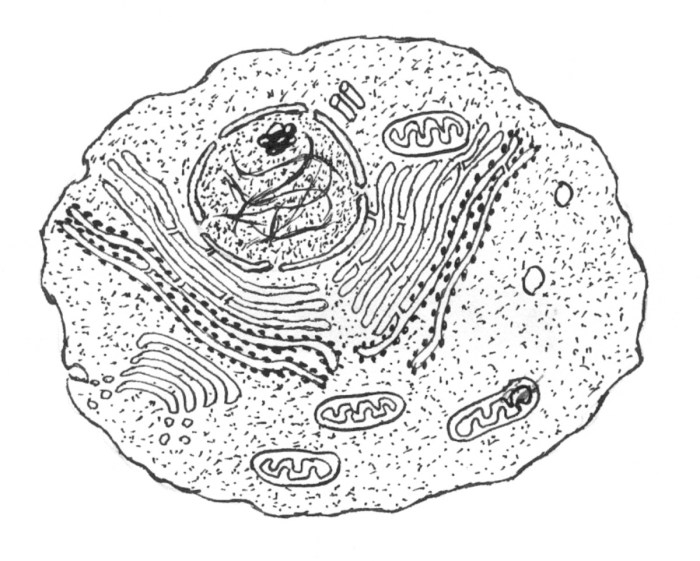
The vibrant hues revealed through staining techniques offer a powerful window into the intricate architecture of the eukaryotic animal cell. By selectively binding to specific cellular components, stains highlight the distribution and morphology of organelles, providing crucial insights into their functions and interrelationships. This allows for a visual appreciation of the cell’s dynamic processes and complex organization.The following section details a hypothetical, yet realistically representative, stained animal cell, emphasizing the correlation between color, structure, and function of its key organelles.
This detailed description serves as a pedagogical tool, aiding in the understanding of cellular components and their visual representation in microscopy.
A Stained Animal Cell: Microscopic Morphology and Color Interpretation
Imagine a microscopic field of view revealing a single, stained animal cell. The cell, roughly spherical with a diameter of approximately 20 micrometers, displays a distinct plasma membrane appearing as a thin, translucent boundary. The cytoplasm, the space enclosed by the membrane, is a light pink hue due to eosin Y staining, a common dye with affinity for cytoplasmic proteins.
Scattered throughout this pink matrix are numerous organelles, each exhibiting unique color and morphology due to differential staining.The nucleus, a large, centrally located ovoid structure, is a deep purple. This intense coloration results from hematoxylin staining, which specifically targets the DNA-rich chromatin within the nucleus. The nucleolus, a smaller, darker purple structure within the nucleus, represents the site of ribosome biogenesis, its darker stain indicating a higher concentration of nucleic acids.The mitochondria, the “powerhouses” of the cell, appear as numerous, elongated, reddish-purple structures.
The Janus Green B stain, often used for mitochondria, imparts this color due to its interaction with the electron transport chain components within the mitochondrial inner membrane. This color intensity reflects the high metabolic activity of these organelles.The endoplasmic reticulum (ER), an extensive network of interconnected membranes, appears as a pale blue-green. This coloration arises from a specific stain targeting the ribosomes attached to the rough ER, highlighting its role in protein synthesis.
Okay, so I’ve been totally engrossed in coloring eukaryotic animal cells lately – it’s surprisingly therapeutic! The detail is amazing, but sometimes I need a break from all those organelles. That’s when I switch to something a little less complex, like the adorable designs found on these cute animal bookmarks coloring pages. Then, refreshed and ready, I can get back to mastering the intricacies of the Golgi apparatus and the endoplasmic reticulum in my cell coloring projects.
The smooth ER, lacking ribosomes, appears less intensely stained, a lighter shade of the same blue-green.The Golgi apparatus, a stack of flattened sacs involved in protein modification and secretion, displays a yellowish-brown color following a specific staining protocol using a dye with an affinity for glycoproteins. This color reflects the abundance of glycosylated proteins processed within this organelle.Lysosomes, small, spherical organelles responsible for cellular waste degradation, appear as small, dark purple dots scattered throughout the cytoplasm.
These organelles are often stained with dyes that bind to acidic hydrolases, reflecting their enzymatic content and role in cellular digestion.
Correlation Between Organelle Color, Function, and Chemical Composition
The color of each organelle in a stained preparation is not arbitrary. It directly reflects the chemical composition of the organelle and its interaction with the specific dye used. For example, the intense purple of the nucleus, due to hematoxylin binding to DNA, indicates the high concentration of nucleic acids within this organelle. Similarly, the reddish-purple of the mitochondria, resulting from Janus Green B’s interaction with the electron transport chain, points to the presence of specific proteins and redox centers crucial for ATP synthesis.
The yellowish-brown of the Golgi apparatus, reflecting the affinity of the stain for glycoproteins, signifies the organelle’s role in post-translational modification and glycosylation of proteins. The overall staining pattern, therefore, provides a visual map of the cell’s biochemical activity and organization.
Methods for Creating Visual Representations: Eukaryotic Animal Cell Coloring
The accurate visualization of eukaryotic animal cells requires meticulous sample preparation and careful microscopic observation techniques. The following sections detail the processes involved in creating visual representations, from sample preparation to the production of labeled diagrams. These methods ensure the creation of clear, informative, and scientifically accurate illustrations of cellular structures.
The successful visualization of cellular structures relies heavily on the proper preparation of the cell sample. This involves several crucial steps, each contributing to the final image quality.
Sample Preparation and Staining
Preparing animal cells for microscopic observation necessitates a multi-step process. First, a sample of animal cells must be obtained. This might involve obtaining a tissue sample from an animal, culturing cells in a laboratory setting, or using commercially available prepared slides. The sample is then thinly spread on a microscope slide to create a single-cell layer, preventing overlapping cells that obscure details.
This spreading can be achieved using various techniques, including gentle smearing or the use of a cytocentrifuge. Following this, the sample undergoes fixation, typically using chemicals like formaldehyde or ethanol, which preserves the cell’s structure and prevents degradation. This is followed by staining. Staining employs dyes that bind to specific cellular components, enhancing their visibility under the microscope.
Hematoxylin and eosin (H&E) staining is a common technique that stains the cell nucleus purple (hematoxylin) and the cytoplasm pink (eosin). Other stains, such as Giemsa or Wright’s stain, are used depending on the specific cellular structures of interest. Finally, a coverslip is carefully placed over the stained sample to protect it and flatten the cells for better observation.
Light Microscopy Observation
Once the sample is prepared, it can be observed using a light microscope. The microscope is initially adjusted to the lowest magnification, ensuring the slide is properly positioned. The coarse focus knob is used to bring the sample into approximate focus, followed by the fine focus knob for precise adjustment. The magnification is gradually increased using the objective lenses (e.g., 4x, 10x, 40x, 100x oil immersion), with each increase requiring further fine-tuning of the focus.
Proper lighting is crucial; the condenser should be adjusted to optimize illumination and contrast. At higher magnifications, immersion oil may be required to improve resolution. Careful observation and recording of the cell structures are essential, noting the shape, size, and staining patterns of different organelles.
Creating a Labeled Diagram, Eukaryotic animal cell coloring
Creating a labeled diagram of a stained eukaryotic animal cell involves a structured approach. First, meticulous observation of the stained cell under the microscope is crucial. Detailed notes should be taken regarding the location, shape, size, and staining characteristics of each organelle. This data forms the basis for the diagram. The diagram can be created either by hand using drawing instruments and colored pencils or markers, or using specialized software such as Adobe Illustrator or BioRender.
If drawing by hand, a pencil sketch is initially created, outlining the cell membrane and major organelles. Colored pencils or markers are then used to accurately represent the staining patterns observed under the microscope. When using software, the cell and its organelles can be drawn using digital tools, and color can be added digitally. Finally, labels are added to each organelle using clear, concise terminology.
The labels should be neatly arranged, avoiding clutter and ensuring that each organelle is clearly identified. A title should be included, indicating the type of cell and the staining method used.
Advanced Staining Techniques and Applications
The visualization of specific cellular components and processes requires techniques beyond basic staining methods. Advanced staining techniques offer unparalleled resolution and specificity, allowing researchers to probe the intricate workings of the eukaryotic animal cell at a molecular level. These methods are crucial for both fundamental biological research and the advancement of medical diagnostics.Advanced staining techniques, such as immunofluorescence and confocal microscopy, represent significant advancements in visualizing cellular structures and processes.
These methods move beyond simple dye-based staining, enabling the targeted labeling and visualization of specific proteins or other molecules within the complex milieu of the cell. This level of detail is essential for understanding cellular function and dysfunction in health and disease.
Immunofluorescence Microscopy
Immunofluorescence microscopy leverages the highly specific binding of antibodies to target antigens within the cell. Fluorescent dyes are conjugated to these antibodies, allowing for the visualization of the target molecule under a fluorescence microscope. The technique’s power lies in its ability to pinpoint the location and distribution of specific proteins, providing insights into their roles in cellular processes.
For instance, immunofluorescence can reveal the localization of a particular receptor protein on the cell membrane, or the distribution of a specific cytoskeletal protein within the cell’s interior. The specificity of antibody-antigen binding ensures that the signal is highly targeted, minimizing background noise and maximizing the clarity of the image. However, the technique requires careful optimization of antibody concentrations and washing steps to prevent non-specific binding and artifacts.
Confocal Microscopy
Confocal microscopy is a powerful imaging technique that uses a pinhole aperture to eliminate out-of-focus light, resulting in significantly improved resolution and image clarity compared to conventional fluorescence microscopy. This technique is particularly valuable when visualizing thick specimens, such as intact tissues or cells in three dimensions. By scanning the sample point-by-point, a detailed three-dimensional reconstruction of the cellular structure can be generated.
For example, confocal microscopy could be used to visualize the intricate network of microtubules within a cell, or to track the movement of organelles in real-time. The high resolution and three-dimensional capabilities of confocal microscopy make it an invaluable tool for studying complex cellular processes and structures. However, the technique is more complex and time-consuming than conventional fluorescence microscopy, and the equipment is relatively expensive.
Comparison of Advanced Staining Techniques
A comparison of immunofluorescence and confocal microscopy highlights their respective strengths and limitations. Immunofluorescence offers high specificity for targeting specific molecules, but its resolution is limited by the diffraction of light. Confocal microscopy, on the other hand, provides significantly improved resolution due to its ability to eliminate out-of-focus light, allowing for the visualization of finer cellular details. However, confocal microscopy does not inherently offer increased specificity in targeting molecules; it enhances the visualization of whatever is labeled by the fluorescent probe.
Thus, the choice of technique depends on the specific research question and the desired level of detail. Both methods are powerful tools, and their combined use can provide a comprehensive understanding of cellular structure and function.
Applications in Biological Research and Medical Diagnostics
Advanced staining techniques are indispensable tools in both biological research and medical diagnostics. In biological research, these techniques are used to study a wide range of cellular processes, including cell division, protein trafficking, signal transduction, and cell-cell interactions. For instance, immunofluorescence is routinely used to study the localization of proteins involved in cell signaling pathways, providing insights into the mechanisms that regulate cellular responses to external stimuli.
In medical diagnostics, these techniques are used to identify and characterize diseases, such as cancer and infectious diseases. For example, immunofluorescence is used to detect the presence of specific pathogens in tissue samples, while confocal microscopy is used to visualize the three-dimensional structure of tumors, providing valuable information for diagnosis and treatment planning. The continued development and refinement of these techniques are crucial for advancing our understanding of biology and improving human health.